Laboratory Testing for Pertussis
'Apology'用'My apologies for the delay'致歉 #生活技巧# #职场沟通技巧# #商务英语口语#
Clinical laboratories can provide diagnostic testing for pertussis. Culture is considered the gold standard. Other tests that can be performed include polymerase chain reaction (PCR) and serology.
Test methods
Clinical laboratories commonly use several types of diagnostic tests to identify Bordetella pertussis:
Culture PCR SerologyDescribed below are advantages and disadvantages for each test method.
The advantages and disadvantages for specimen collection for each test method. Method Advantages Disadvantages Culture Gold standard for pertussis diagnosis The only 100% specific method for identification Better specificity than PCR Takes up to 7 days to obtain results PCR Most rapid test available Excellent sensitivity PCR assays that include multiple target sequences allow for speciation among Bordetella species Tests can vary in specificity High sensitivity increases risk of false-positivity but best practices can reduce risk of inaccurate results Serology Can be performed much later in the course of disease than culture and PCR Some commercially available tests have unproven or unknown clinical accuracyImportant consideration
Interpret PCR results with clinical symptoms and epidemiological information. In the setting of an outbreak, culture confirmation of at least one case should occur.
Optimal timing for diagnostic testing
Described below is the optimal timing for when to use each test method.
Culture Collect during first 2 weeks of illness following cough onset when viable bacteria are still present Sensitivity decreases after the first 2 weeks, which increases risk of false-negative results PCR Use up to 3 to 4 weeks following cough onset After fourth week of cough, amount of bacterial DNA in nasopharynx rapidly diminishes, increasing risk of a false-negative result Serology Use 2 to 8 weeks following cough onset for optimal results due to highest antibody titers Can be used up to 12 weeks following cough onset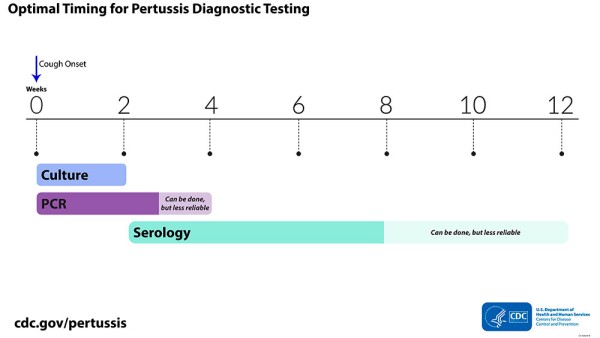
Visual depiction of the optimal timing for diagnostic testing (weeks).
CDC serologic test
CDC and the Food and Drug Administration developed a serologic assay that has been useful for confirming diagnosis, especially during suspected pertussis outbreaks. A few state public health laboratories have included this assay as part of their testing regimen for pertussis. This assay is also available for research or surveillance purposes through CDC's test directory.
CDC evaluation of commercially available assaysCommercially, there are several different serologic tests used in the United States with unproven or unknown clinical accuracy. CDC performed an evaluation of several commercially available assays in the United States and found great variability between them, depending on the antigen or antibody used and whether they were calibrated to an international reference serum. Analysis found the most promising assays were those that measured IgG antibodies against pertussis toxin only and were calibrated to a reference standard.
Specimen collection
Obtaining a nasopharyngeal (NP) swab or aspirate
Healthcare providers should obtain an NP swab or aspirate from everyone with suspected pertussis.
Using specimen for cultureDirectly plate collected NP swab or immediately place into transport medium. Plating should occur within 24 hours of NP swab or aspirate collection.
Proper technique for obtaining an NP specimen for isolation
A properly obtained NP swab or aspirate is needed for optimal diagnostic results. The same specimen can be used both for culture and PCR.
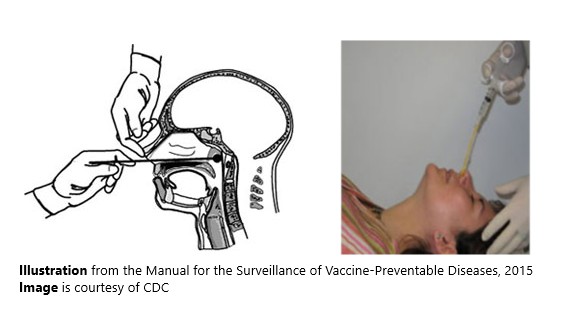
Proper technique is needed for optimal diagnostic results.
SOURCE:
From the Manual for the Surveillance of Vaccine-Preventable Diseases, 2015.
Submitting specimens
Specimen acceptance criteria
CDC only accepts various specimen types for B. pertussis testing from public health laboratories and other federal agencies.
Specimens from private healthcare providers and institutions must be submitted to a public health department laboratory for appropriate processing.
Specimen, documentation, packaging, and shipping
Specimen requirements vary by the specific test requested.
The following links provide information on specimen, documentation, packaging, and shipping requirements:
B. pertussis and related species detection and identification B. pertussis serologyResources
Pertussis Outbreaks CDC's Pertussis and Diphtheria Laboratory CDC Surveillance Manual's Chapter on Pertussis – Laboratory TestingApr. 2, 2024Was this page helpful?
How would you rate this information? Not helpful Very helpful
网址:Laboratory Testing for Pertussis https://klqsh.com/news/view/103099
相关内容
Laboratory Testing for CMV and Congenital CMVLaboratory Testing for Epstein
Clinical Testing and Diagnosis for Lyme Disease
Fit Testing
Bing Tests Related Search Interfaces
MandalaIt.com is for sale
GobyPro.com is for sale
Kitchen Flooring Ideas: The Ultimate UK Style Guide for 2025
Release notes for Bing Custom Web Search API
高考英语必备—高考英语满分作文解析及写作素材积累话题 01 学校生活与课外活动

